Exemestane API Market Expands at 6.1% CAGR, Reaching USD 2.42 Billion by 2035
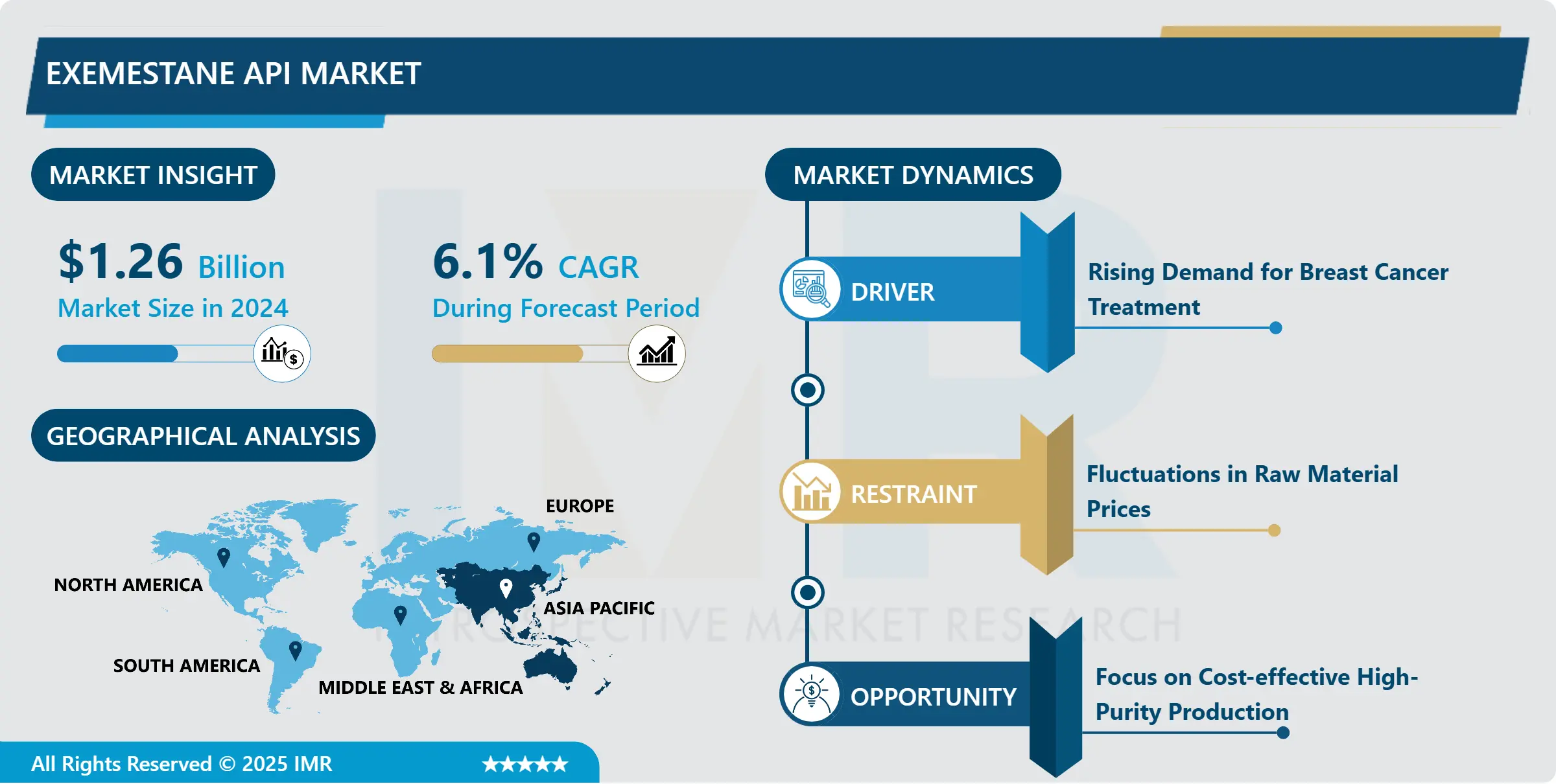
“According to a new report published by Introspective Market Research, Exemestane API Market by Purity, Downstream Industry, and Therapeutic Application, The Global Exemestane API Market Size Was Valued at USD 1.26 Billion in 2024 and is Projected to Reach USD 2.42 Billion by 2035, Growing at a CAGR of 6.1%.”
Exemestane Active Pharmaceutical Ingredient (API) is a potent, irreversible steroidal aromatase inhibitor primarily used in the treatment of hormone receptor-positive breast cancer in postmenopausal women. As a third-generation aromatase inhibitor, Exemestane works by permanently inactivating the aromatase enzyme, which is responsible for estrogen production, thereby starving estrogen-dependent tumors of the hormone needed for their growth. This mechanism offers a distinct therapeutic advantage over traditional hormonal alternatives that may only offer temporary suppression.
The primary use of Exemestane API is in the manufacturing of its final drug formtabletswhich are a cornerstone in sequential endocrine therapy protocols for both early and advanced breast cancer management. Its established efficacy, favorable safety profile, and strategic role in oncology therapeutics have cemented its position in the global pharmaceutical landscape. The rising prevalence of breast cancer globally and the subsequent growing demand for effective, targeted hormonal treatments are key factors propelling the demand for high-quality Exemestane API, driving substantial growth in the market.
Market Segmentation:
The Exemestane API Market is segmented into Purity, Downstream Industry, and Therapeutic Application. By Purity, the market is categorized into (Purity $\geq 98\%$, Purity $\geq 99\%$). By Downstream Industry, the market is categorized into (Exemestane Capsules, Exemestane Tablets, Others). By Therapeutic Application, the market is categorized into (Breast Cancer Treatment, Other Applications).
Growth Driver:
The primary growth driver for the Exemestane API market is the escalating global incidence of breast cancer, particularly among the postmenopausal population who are the primary recipients of this treatment. Breast cancer is the most common cancer among women worldwide, and as global life expectancy rises, so does the population segment most susceptible to hormone-receptor-positive variants of the disease. Consequently, the demand for effective hormonal therapies, such as those utilizing Exemestane API, sees a proportional increase. Furthermore, the inclusion of Aromatase Inhibitors (AIs) in standard clinical guidelines, often recommending them as first-line or adjuvant therapy, solidifies the drug’s commercial trajectory and boosts API production volumes across major manufacturing hubs in Asia-Pacific and other regions.
Market Opportunity:
A significant market opportunity lies in the expansion of generic drug manufacturing and formulation in emerging economies. Following the patent expiry of the branded formulation of Exemestane (Aromasin) in many jurisdictions, there has been a surge in generic production. Emerging markets, especially in Asia-Pacific, offer substantial cost advantages in API and finished dosage formulation due to lower labor costs and supportive government initiatives for local pharmaceutical production. This environment fosters a shift towards cost-effective healthcare solutions, where affordable Exemestane generics increase patient access, driving high-volume demand for the API. Strategic alliances between international and local manufacturers to optimize supply chain and reduce manufacturing costs represent a key area for high-profit market penetration.
Detailed Segmentation:
Title: Exemestane API Market, Segmentation
The Exemestane API Market is segmented on the basis of Purity, Downstream Industry, and Therapeutic Application.
Therapeutic Application
The Therapeutic Application segment is further classified into Breast Cancer Treatment and Other Applications. Among these, the Breast Cancer Treatment sub-segment accounted for the highest market share in 2024. Exemestane’s well-established and primary role as a crucial third-generation aromatase inhibitor is the reason for this dominance. It is an essential component in managing hormone-dependent breast cancer in postmenopausal women, used for both adjuvant therapy (after initial treatment) and for advanced disease. Its proven efficacy in preventing cancer recurrence and progression, coupled with its distinct irreversible binding mechanism to the aromatase enzyme, makes it a favored compound in international oncology guidelines. The high global prevalence of estrogen receptor-positive breast cancer ensures sustained, high-volume demand for Exemestane API in this therapeutic area, leading to its segment leadership.
Downstream Industry
The Downstream Industry segment is further classified into Exemestane Capsules, Exemestane Tablets, and Others. Among these, the Exemestane Tablets sub-segment accounted for the highest market share in 2024. The tablet dosage form is the standard and widely accepted route of administration for Exemestane, with the most common strength being 25 mg. The drug was originally developed and launched as an oral tablet (Aromasin), and subsequent generic versions have predominantly adopted this format for market continuity, patient familiarity, and ease of mass production. The regulatory approvals and clinical trial data are primarily based on the tablet form, which reinforces its preferential use by oncologists globally. The cost-effectiveness of mass-producing the solid oral tablet further contributes to its dominant market share in the overall downstream industry.
Some of The Leading/Active Market Players Are-
· Pfizer Inc. (United States)
· Cipla Limited (India)
· Qilu Pharmaceutical (China)
· Teva Pharmaceutical Industries Ltd. (Israel)
· Dr. Reddy’s Laboratories Ltd. (India)
· Aurobindo Pharma (India)
· Axplora (France)
· Lupin Limited (India)
· Sun Pharmaceutical Industries Ltd. (India)
· Symbiotec Pharma Lab Pvt. Ltd. (India)
· Sterling S.p.A. (Italy)
· Trifarma S.p.A. (Italy)
· Coral Drugs Private Limited (India)
· ScinoPharm Taiwan Ltd. (Taiwan)
· and other active players.
Key Industry Developments
New Facility Investment
In June 2024, a major Indian API manufacturer, Divi's Laboratories, announced a significant capital expenditure plan to expand its high-potency API (HPAPI) and oncology-related manufacturing capacities. This investment is aimed at capitalizing on the growing demand for cancer treatment raw materials.
This expansion is strategically positioned to meet the increasing global need for key oncology APIs, including Exemestane, which falls under the category of complex, high-value molecules. By boosting manufacturing scale and adhering to stringent international regulatory standards, the company aims to secure long-term supply contracts, particularly in the highly lucrative regulated markets of North America and Europe, thereby strengthening its market share.
Regulatory Approval for Generic
In January 2025, the U.S. Food and Drug Administration (FDA) granted final approval for a new generic version of Exemestane tablets (25 mg) submitted by Zydus Lifesciences for the treatment of early and advanced breast cancer in postmenopausal women.
This regulatory action immediately adds another low-cost alternative to the U.S. market, increasing patient access to this critical medication. The introduction of new generic players intensifies price competition among API suppliers, compelling them to focus on manufacturing efficiency and quality compliance to remain competitive and capture larger procurement volumes from finished-dosage manufacturers.
Key Findings of the Study
· Dominant segments: The Breast Cancer Treatment application segment and the Exemestane Tablets downstream industry segment are the dominant market sectors.
· Leading regions: North America holds a substantial share due to advanced healthcare infrastructure, while Asia-Pacific is the fastest-growing region driven by local manufacturing and cost-competitive generic production.
· Key growth drivers: The primary driver is the rising global prevalence of hormone-receptor-positive breast cancer in the postmenopausal demographic.
· Market trends: A major trend is the strategic focus on generic API manufacturing following the patent expiration of the branded drug.
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Exemestane API Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global Exemestane API industry.
More Info:- https://introspectivemarketresearch.com/reports/exemestane-api-market/
📞 Contact Us: Introspective Market Research Pvt. Ltd.
Phone: +91-91753-37569
Email: sales@introspectivemarketresearch.com
Web: www.introspectivemarketresearch.com
- Sports
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spiele
- Gardening
- Health
- Startseite
- Literature
- Music
- Networking
- Andere
- Party
- Shopping
- Theater
- Wellness


