Gene Therapy for Rare Disease Market to Grow at a CAGR of 35.5% Through 2032
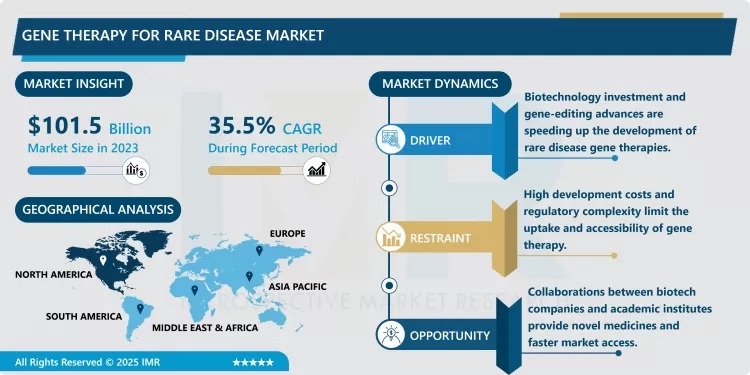
“According to a new report published by Introspective Market Research, Gene Therapy for Rare Disease Market by Therapy Type, Vector Type, and Indication, The Global Gene Therapy for Rare Disease Market Size Was Valued at USD 101.5 Billion in 2023 and is Projected to Reach USD 2,869.54 Billion by 2032, Growing at a CAGR of 35.5% from 2024–2032.”
Gene therapy for rare diseases represents a transformative approach to treating genetic disorders by addressing the root cause at the molecular level. This innovative therapeutic method involves the introduction, removal, or alteration of genetic material within a patient’s cells to correct defective genes responsible for rare and inherited diseases. Compared to traditional treatments that focus on symptom management, gene therapy offers long-term or potentially curative outcomes.
The market has witnessed significant traction due to advancements in viral vector technologies, genome editing tools such as CRISPR-Cas9, and increasing regulatory approvals for gene-based treatments. These therapies are being widely applied across indications such as spinal muscular atrophy, hemophilia, inherited retinal diseases, and metabolic disorders.
Growing investments from biotechnology companies, favorable orphan drug regulations, and rising awareness of rare diseases are accelerating market expansion. Additionally, increasing collaboration between academic institutions and pharmaceutical companies is fostering innovation and commercialization within the global gene therapy ecosystem.
Market Segmentation
The Gene Therapy for Rare Disease Market is segmented into Therapy Type, Vector Type, and Indication.
By Therapy Type, the market is categorized into In Vivo Gene Therapy, Ex Vivo Gene Therapy, and Genome Editing Therapies.
By Vector Type, the market is categorized into Viral Vectors, Non-Viral Vectors, and Hybrid Vectors.
By Indication, the market is categorized into Neurological Disorders, Hematological Disorders, Metabolic Disorders, Ophthalmic Disorders, and Others.
Growth Driver
The primary growth driver of the gene therapy for rare disease market is the rising prevalence of rare genetic disorders coupled with unmet medical needs. Thousands of rare diseases currently lack effective treatments, driving demand for innovative therapeutic solutions. Advances in genetic research and sequencing technologies have improved disease diagnosis and target identification, enabling the development of highly personalized gene therapies. Furthermore, strong regulatory support through orphan drug designations, accelerated approval pathways, and extended market exclusivity is encouraging pharmaceutical companies to invest heavily in gene therapy research and development.
Market Opportunity
A major market opportunity lies in the expanding pipeline of gene therapies and increasing commercialization of approved products. Rapid technological advancements in vector engineering, gene delivery methods, and manufacturing scalability are reducing development costs and improving safety profiles. Emerging markets also present untapped potential due to improving healthcare infrastructure and rising awareness of rare diseases. Additionally, partnerships between biotech firms and large pharmaceutical companies are expected to accelerate clinical trials, expand global reach, and create long-term growth opportunities for market participants.
Detailed Segmentation
Gene Therapy for Rare Disease Market, Segmentation
The Gene Therapy for Rare Disease Market is segmented on the basis of Therapy Type, Vector Type, and Indication.
Therapy Type
The Therapy Type segment is further classified into In Vivo Gene Therapy, Ex Vivo Gene Therapy, and Genome Editing Therapies. Among these, the In Vivo Gene Therapy sub-segment accounted for the highest market share in 2023. In vivo therapies enable direct delivery of genetic material into the patient’s body, offering simplified treatment protocols and broader clinical applicability. This approach is widely used for neurological and ophthalmic disorders, driving its dominance in the market due to higher adoption and increasing regulatory approvals.
Vector Type
The Vector Type segment is further classified into Viral Vectors, Non-Viral Vectors, and Hybrid Vectors. Among these, the Viral Vectors sub-segment accounted for the highest market share in 2023. Viral vectors such as adeno-associated viruses (AAVs) and lentiviruses are preferred due to their high transduction efficiency and stable gene expression. Continuous improvements in vector safety and targeting capabilities are further strengthening their position in gene therapy applications.
Some of The Leading/Active Market Players Are-
• Novartis AG (Switzerland)
• F. Hoffmann-La Roche Ltd. (Switzerland)
• Pfizer Inc. (USA)
• Gilead Sciences, Inc. (USA)
• Bluebird Bio, Inc. (USA)
• Sarepta Therapeutics, Inc. (USA)
• Spark Therapeutics, Inc. (USA)
• UniQure N.V. (Netherlands)
• Amgen Inc. (USA)
• BioMarin Pharmaceutical Inc. (USA)
• CRISPR Therapeutics (Switzerland)
• Regenxbio Inc. (USA)
• Orchard Therapeutics (UK)
• Sangamo Therapeutics, Inc. (USA)
• Voyager Therapeutics, Inc. (USA)
and other active players.
Key Industry Developments
In March 2024, a leading biotechnology company announced positive Phase III clinical trial results for a gene therapy targeting a rare neurological disorder.
The trial demonstrated significant functional improvement with a favorable safety profile, strengthening the company’s regulatory submission and boosting investor confidence in gene-based treatments.
In September 2023, a global pharmaceutical firm received regulatory approval for a novel gene therapy for a rare hematological disease.
This approval marked a major milestone, expanding treatment options for patients and reinforcing the growing acceptance of gene therapy as a mainstream therapeutic solution.
Key Findings of the Study
• In vivo gene therapy dominates the market due to higher clinical adoption
• Viral vectors remain the leading delivery method
• North America holds the largest market share
• Strong R&D investments and orphan drug incentives drive growth
• Expanding pipelines signal robust future opportunities
More Info:- https://introspectivemarketresearch.com/reports/gene-therapy-for-rare-disease-market/
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Gene Therapy for Rare Disease Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global Hormone Replacement Therapy Market industry.
📞 Contact Us
Introspective Market Research Pvt. Ltd.
📞 Phone: +91-91753-37569
✉️ Email: sales@introspectivemarketresearch.com
🌐 Web: www.introspectivemarketresearch.com
- Sports
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Игры
- Gardening
- Health
- Главная
- Literature
- Music
- Networking
- Другое
- Party
- Shopping
- Theater
- Wellness


